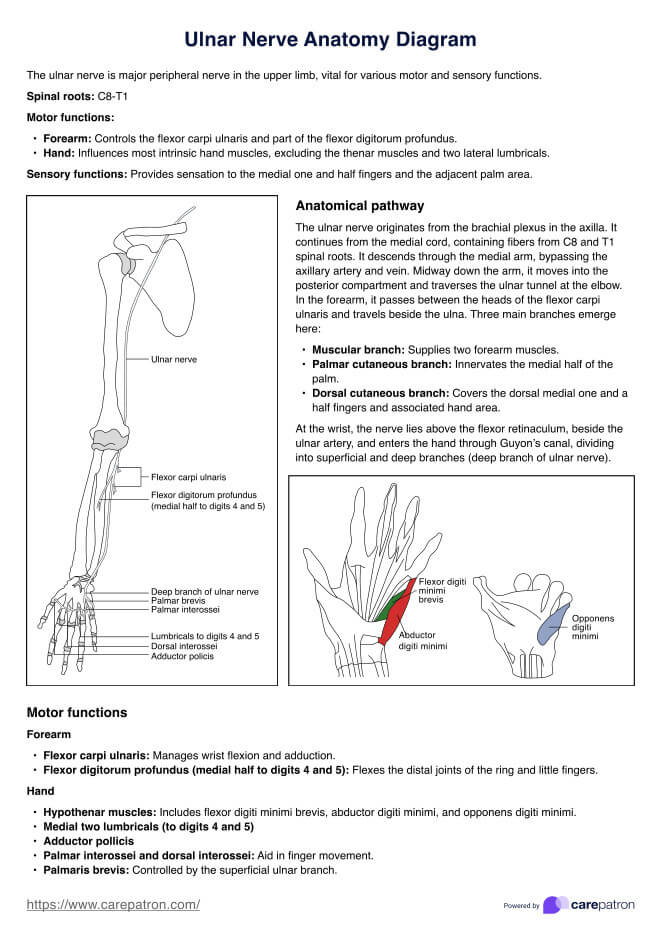
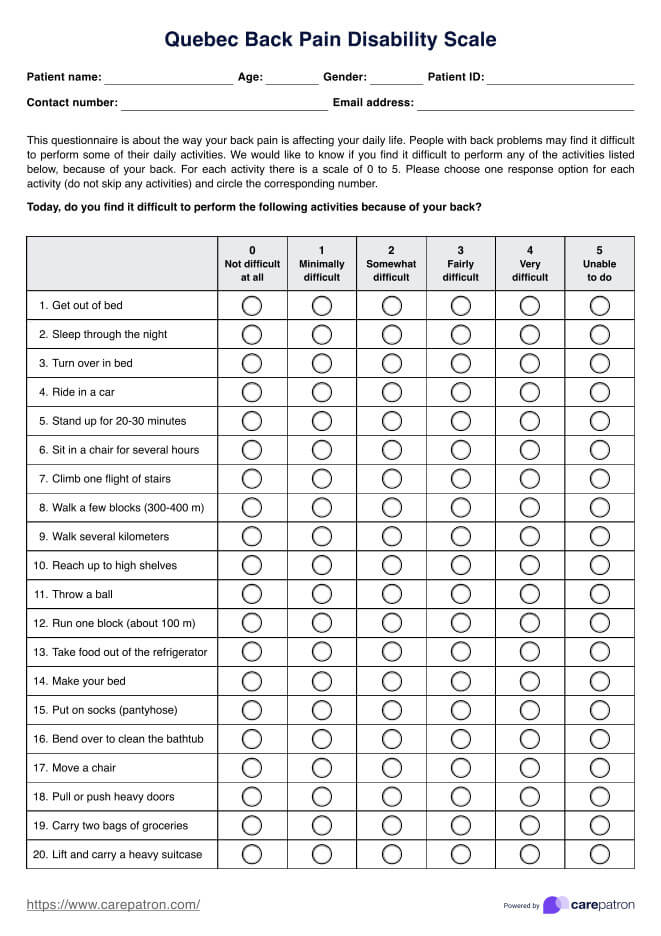
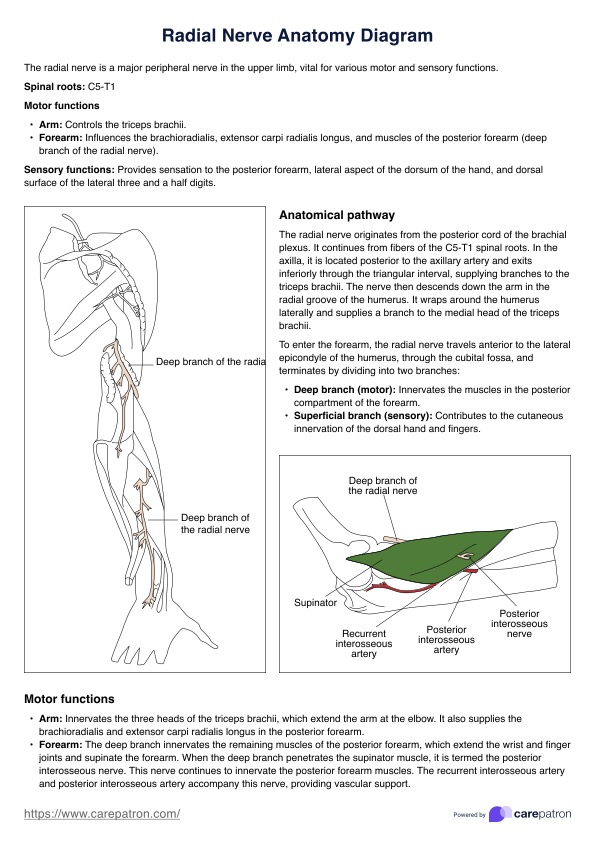
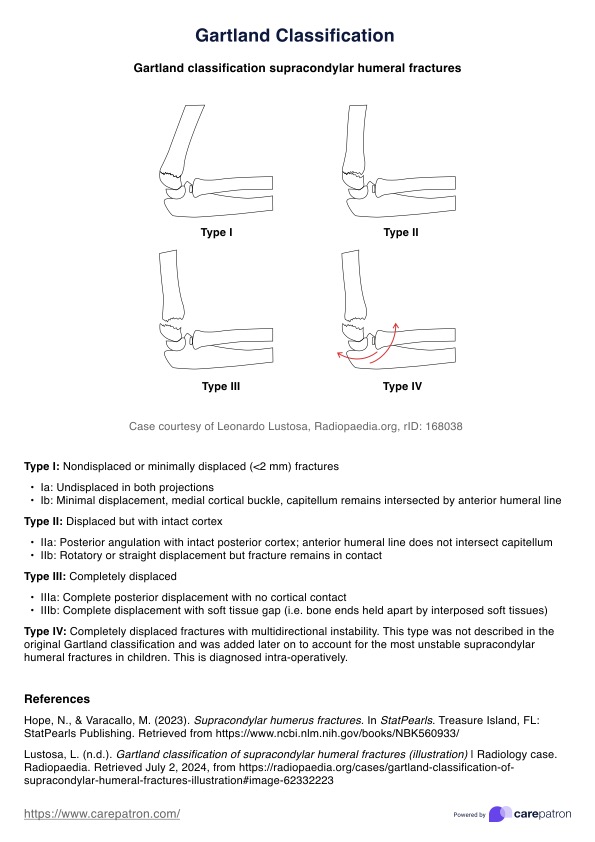
Informed Consent Form
Ensure clarity and transparency with our concise, informed consent form, empowering your clients to make informed decisions regarding their participation.


What is an Informed Consent Form?
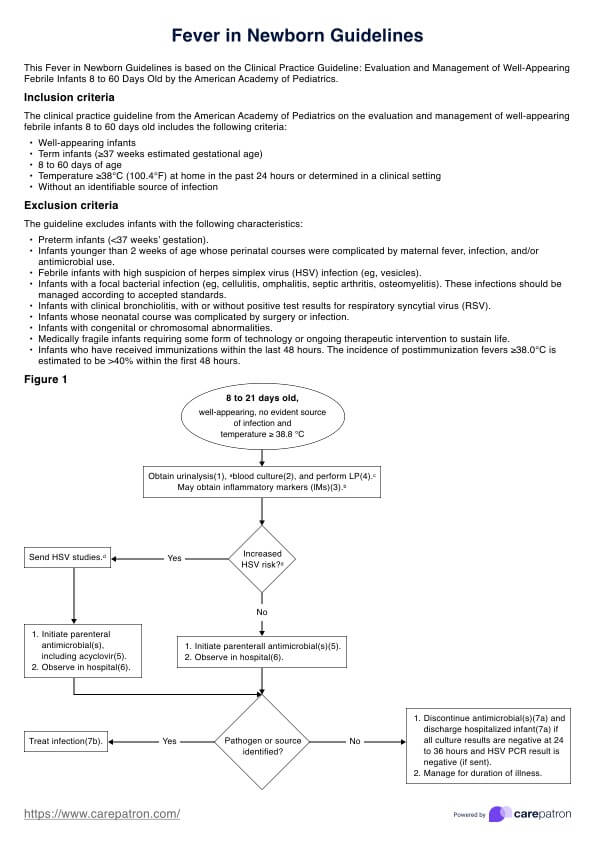
Informed consent is crucial in various fields, including healthcare, research, and legal contexts. It aims to ensure that individuals fully understand the nature, risks, benefits, and implications of their involvement in a particular activity or procedure before consenting.
Research
In research, Informed Consent Forms play a vital role in protecting the rights and welfare of participants. These forms detail the purpose of the study, procedures involved, potential risks and benefits, confidentiality measures, and the participant’s rights. Researchers must ensure that participants understand the information required by federal regulations and provided by the researchers before voluntarily agreeing to participate without coercion or undue influence.
Legal
Legal contexts, such as contracts and agreements, also employ informed consent forms. These documents inform individuals about a particular transaction or legal process’s terms, conditions, rights, and obligations. Individuals acknowledge their understanding and agreement to the specified terms by signing the form.
Healthcare
In healthcare, Informed Consent Forms communicate information about medical procedures, surgeries, treatments, or interventions. Patients are encouraged to ask questions and clarify doubts before signing the form, ensuring their autonomy and correct decision-making about their healthcare.
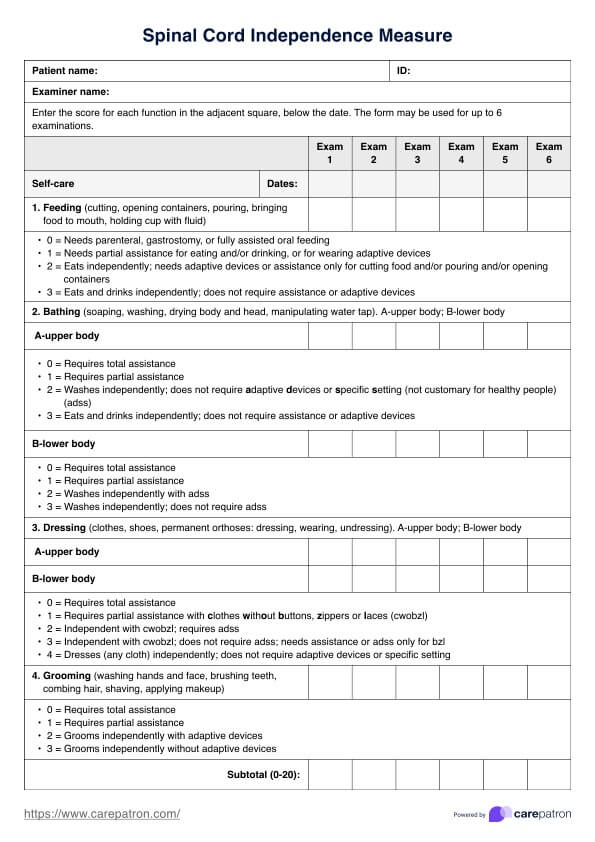
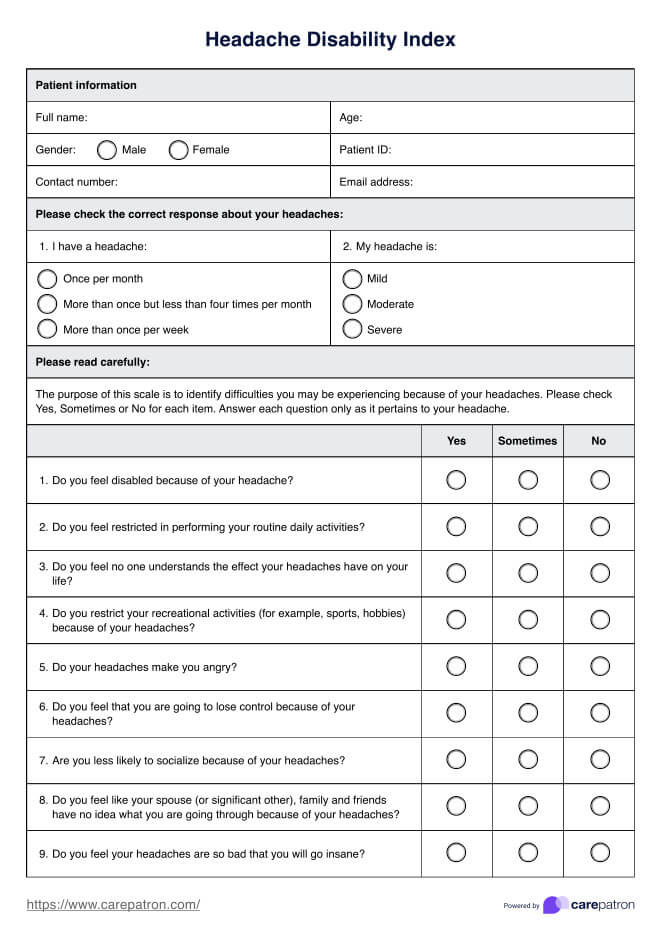
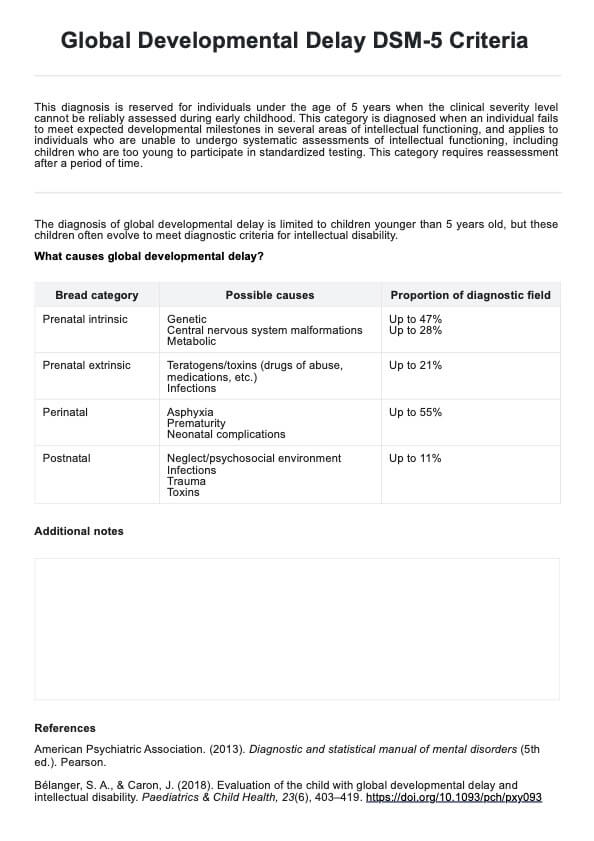
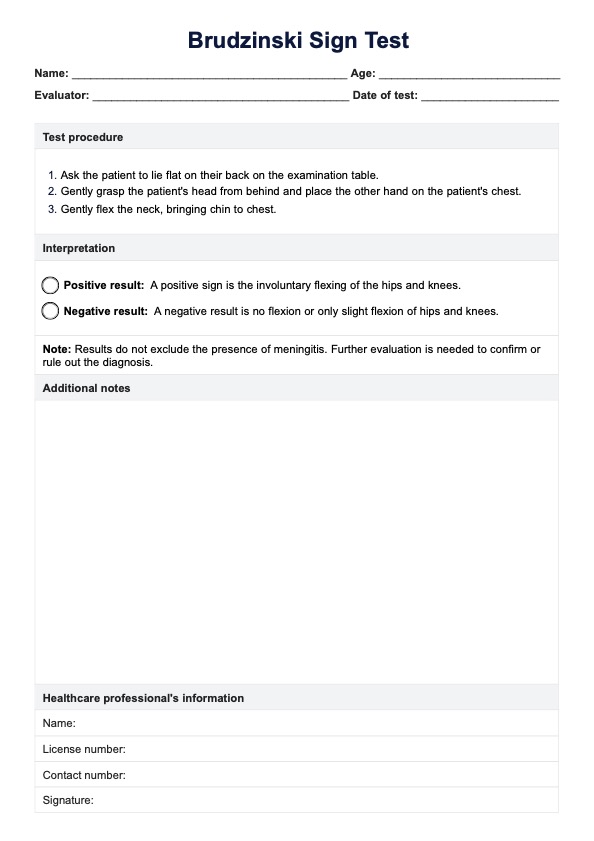
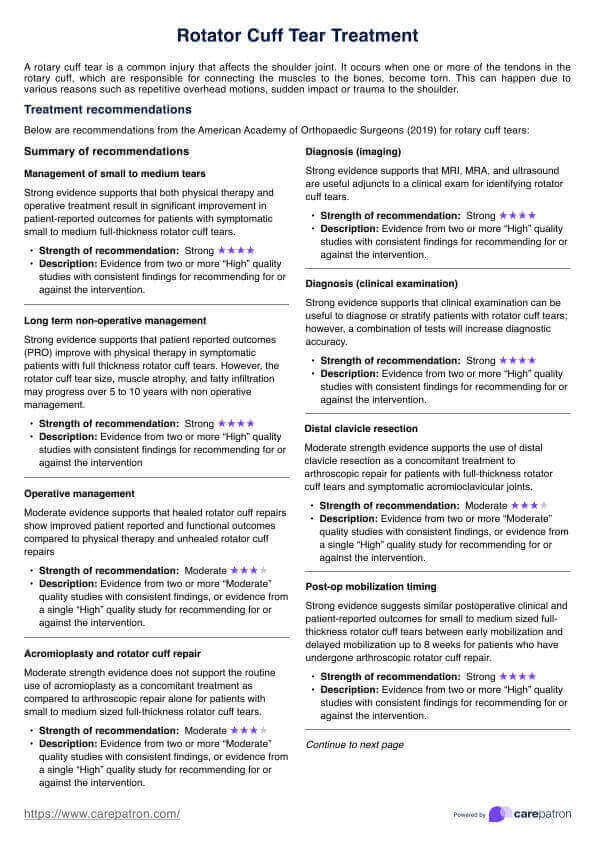
Since this is a guide written to help healthcare practitioners give you an idea of the content of healthcare-informed Consent Forms, here are examples of two of the most commonly used ones:
Informed Consent Form Template
Informed Consent Form Example
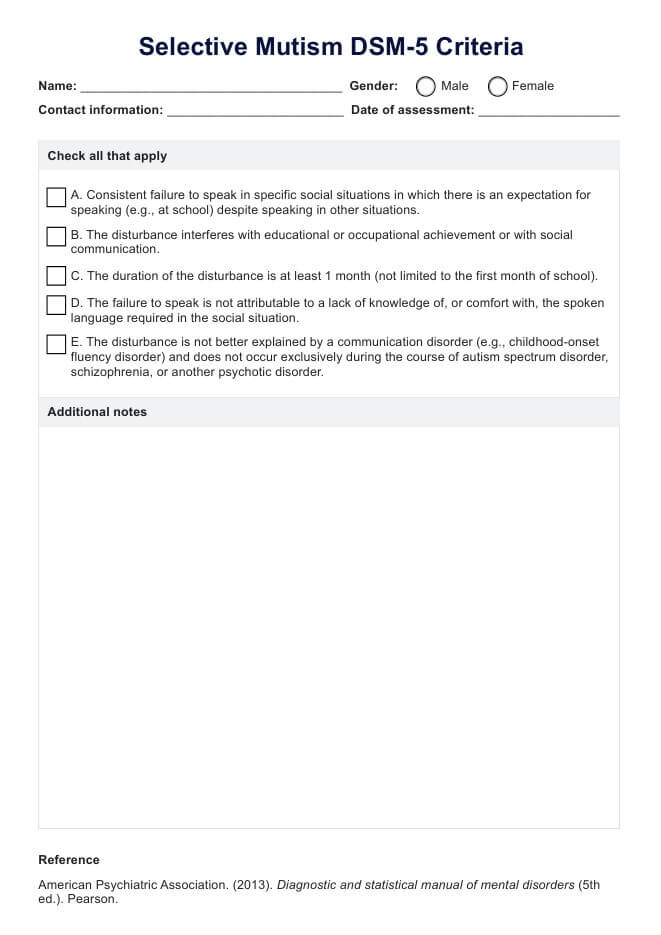
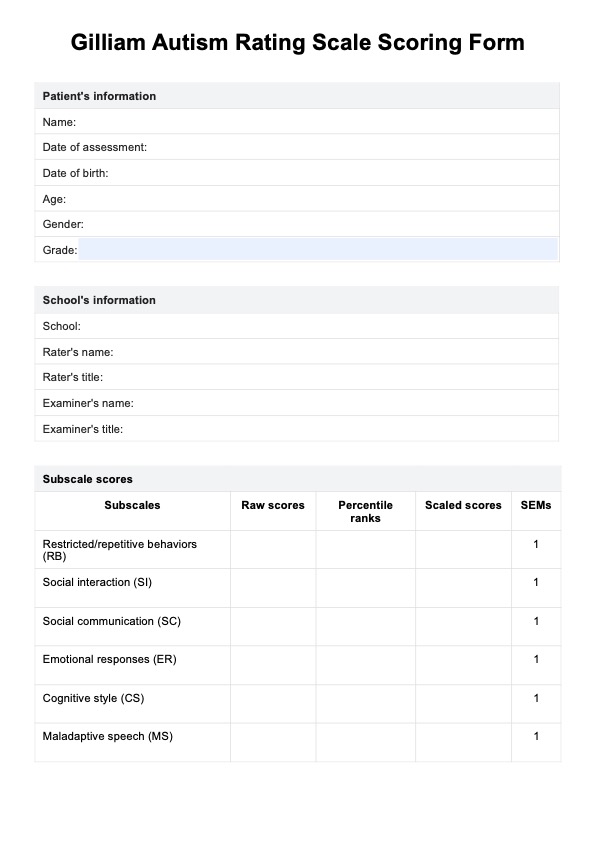
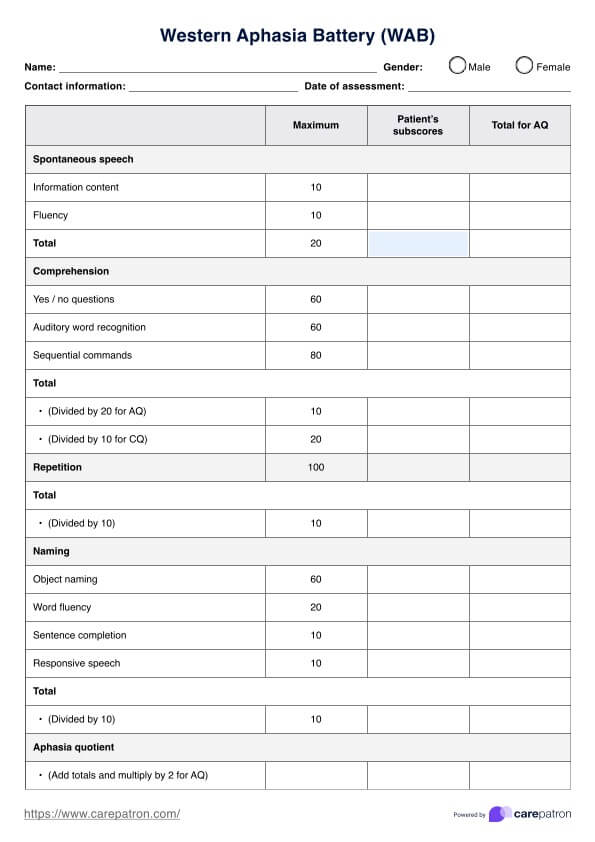
Overview of a psychology Informed Consent Form
A psychology-Informed Consent Form is a very important document that outlines the terms and conditions of psychological therapy and sets the framework for the therapeutic relationship between the therapist and the client. The document ensures that the client is fully informed of the nature of therapy and is aware of the possible risks and benefits of participating in therapy during the ongoing process of therapy.
The consent includes several key components, such as the nature of the therapy, what they can expect from the therapeutic process, confidentiality, risks and benefits, therapist qualifications and fees, and consent to treatment.
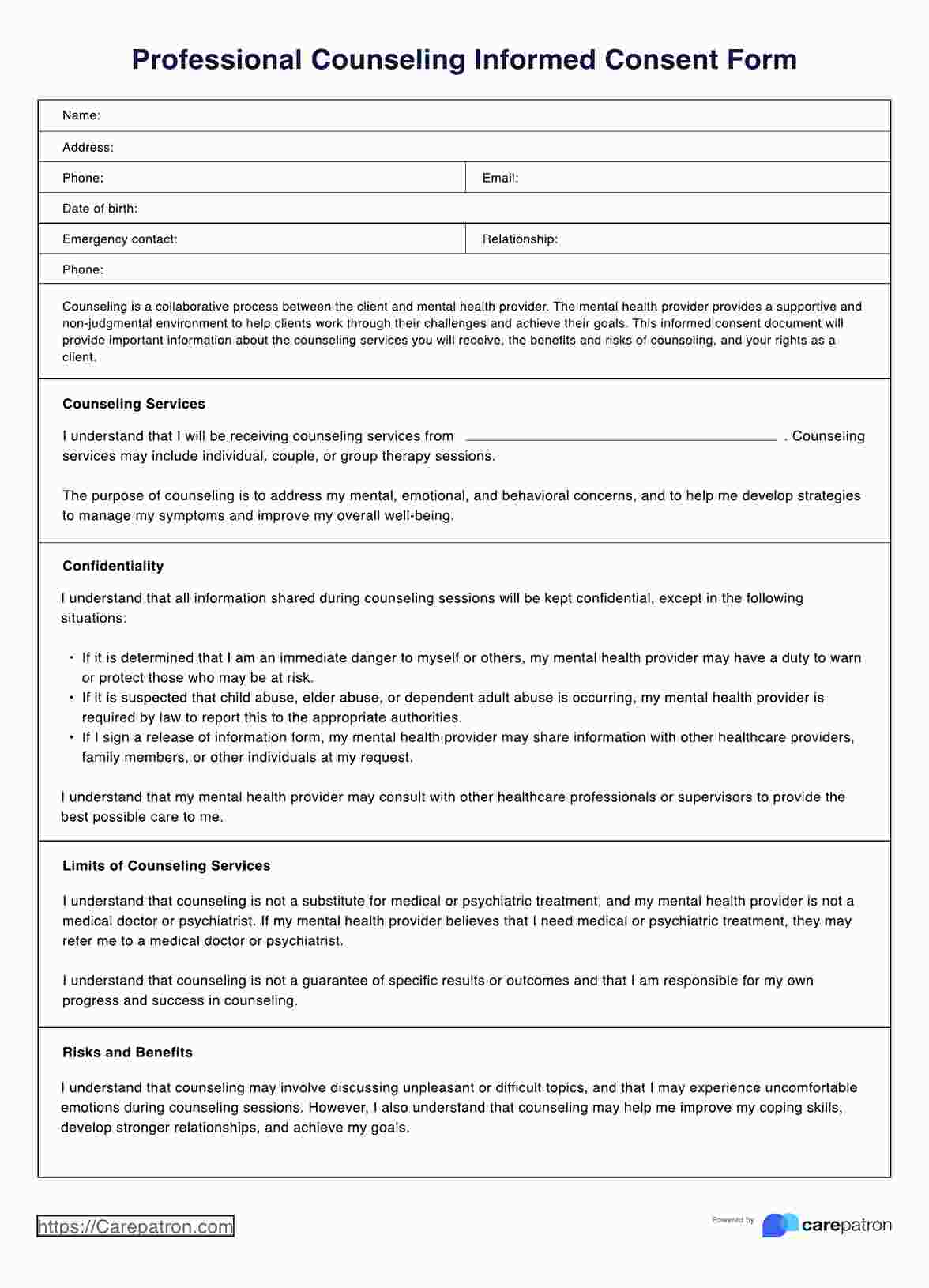
Overview of the counseling Informed Consent Form
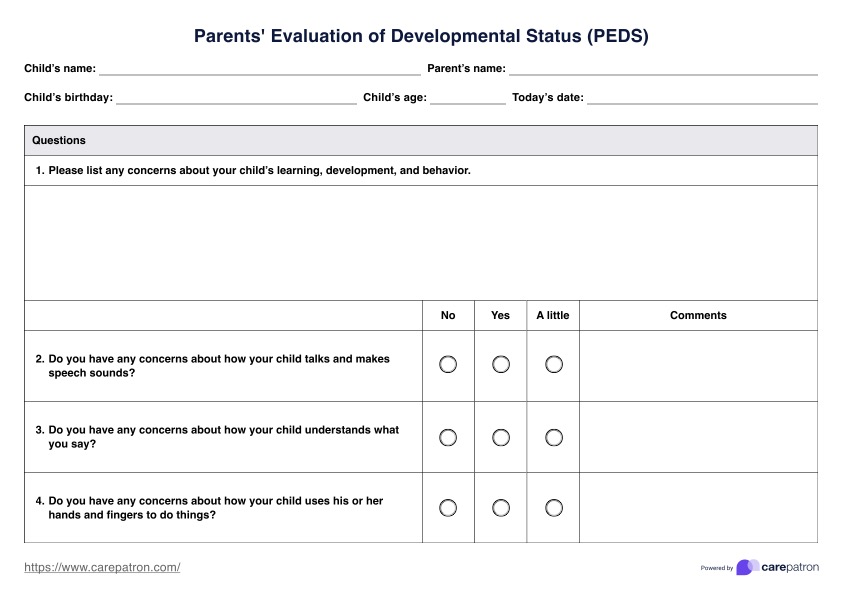
In counseling, healthcare practitioners must always obtain an informed consent document from their clients whether they are counseling for the first time or have received counseling services. Doing so ensures that the prospective participants are fully informed about the counseling process, their rights, and their role.
Aside from ethical and legal reasons, obtaining informed consent is crucial because a new counselor may have a different approach or philosophy than one's previous counselor, and informed consent ensures that patients clearly understand what they can expect.
Typically, counseling informed consent templates describe services and information on voluntary participation, client/therapist involvement, guarantees, meetings, and length of therapy.
How does the informed consent process work?
This printable Informed Consent Form template is a straightforward process involving several steps. Here is a breakdown of how it works:
Step 1: Introduction and obtaining patient information
Begin by introducing the document as an Informed Consent Form, including the name of the healthcare provider or organization and the date of consent. Then, fill in the patient’s full name and other required identification details.
Step 2: Go over the informed consent document
Go over the following required consent elements of the document in simple and easily understandable language:
- Purpose and nature of treatment or procedure: Clearly explain the treatment or procedure the patient will undergo, detailing its purpose, nature, and goals.
- Risks and potential complications: List the potential risks and complications associated with the treatment or procedure, ensuring that common and rare occurrences are covered.
- Expected benefits: Describe the anticipated benefits/improvements that can reasonably be expected from the treatment or procedure and highlight any positive outcomes.
- Alternatives: Present any relevant alternative procedures and treatment options, if applicable, along with their benefits, risks, and limitations.
- Questions and clarifications: Encourage the patient to ask questions or seek clarification about the treatment or procedure.
- Voluntary consent: Emphasize that the patient’s consent is voluntary, and they have the right to refuse or withdraw their consent at any time without any negative impact on their future care.
- Confidentiality and data protection: Explain how patient information will be handled, ensuring confidentiality and adherence to privacy regulations.
- Financial considerations: If relevant, it is strongly advised that you outline any costs, insurance coverage, or payment arrangements associated with the treatment or procedure.
- Follow-up care and aftercare: Describe any necessary follow-up appointments, post-procedure instructions, or ongoing care requirements.
Step 3: Have the patient sign
Provide spaces for the patient’s signature and the date of signing. If required, include a witness’s signature as well. The signatures indicate that the patient has read and understood the information and has had the opportunity to ask questions.
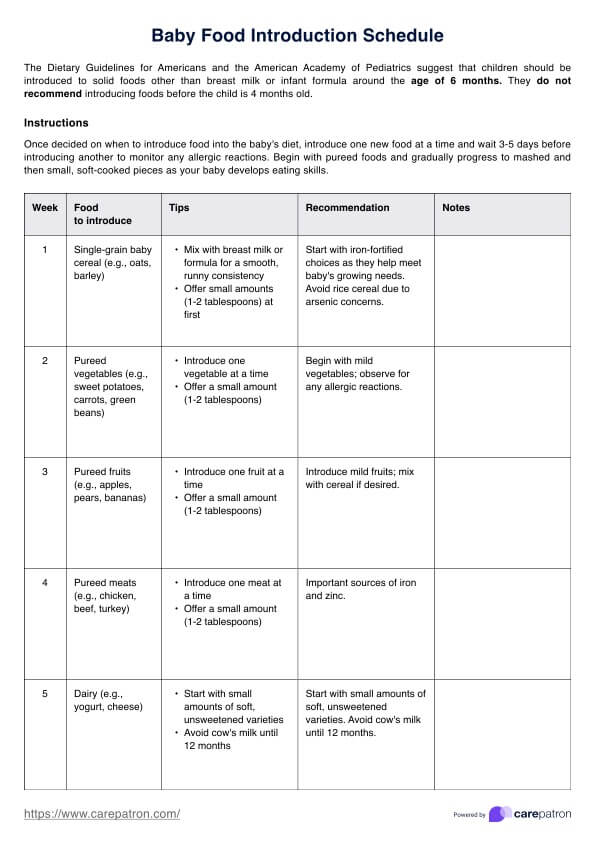
Informed Consent Form example (sample)
The example informed consent form PDF gives patients essential information about their healthcare choices. It covers the purpose and nature of the treatment, potential risks and benefits, alternative options, confidentiality, financial considerations, and the patient’s right to ask questions. The informed consent document should be written in plain language, include specific required elements, and be modified to fit the specific practice or agency. It is also recommended to have a colleague or friend review the document for comprehension and involve legal review to ensure compliance with regulations and statutes. The form emphasizes voluntary consent and the patient’s ability to refuse or withdraw consent.
By signing an Informed Consent Form, patients acknowledge their understanding of the provided information and their agreement to proceed. This concise document ensures transparency, empowers patients to make informed decisions, and protects their rights and welfare throughout their healthcare journey.
Download this Informed Consent Form example (sample) here:

When would you use this template?
This template for an Informed Consent Form can be utilized in various healthcare settings and scenarios where obtaining informed consent from patients is essential. Here are some instances where this template can be handy:
- Medical procedures and surgeries: Healthcare practitioners can use this template when explaining invasive procedures, surgeries, or medical interventions to patients so they understand the nature of the procedure, associated risks, etc.
- Experimental or investigational treatments: In cases where patients are being offered experimental or investigational treatments, this template becomes crucial as it helps practitioners communicate the experimental nature of the treatment, potential risks, benefits, and any uncertainties involved.
- Clinical trials and research studies: Researchers conducting clinical trials, or even exempt research, can utilize this template to obtain informed consent from participants to provide participants with detailed information on the study's purpose, confidentiality measures, etc.
- Mental health treatments: Mental health practitioners can use this template when obtaining consent for various treatments, such as psychotherapy, medication management, or electroconvulsive therapy (ECT), to explain their treatment approach, alternative options, etc.
- Genetic testing and counseling: In the field of genetics, this template can be employed by genetic counselors and healthcare providers offering genetic testing to help them understand the test's purpose, potential implications of the results, privacy concerns, and more.
- Surgical or aesthetic procedures: Surgeons, cosmetic surgeons, and aesthetic practitioners can utilize this template to obtain informed consent for surgical or non-surgical procedures to aid them with understanding the potential risks, necessary post-procedure care, etc.
Benefits
Utilizing this free Informed Consent Form template offers several benefits for healthcare practitioners and patients. Here are some key advantages:
- Clarity and comprehensive information: The template helps healthcare practitioners provide clear and comprehensive information about the treatment, procedure, or study, ensuring patients understand what they consent to.
- Empowerment and informed decision-making: By presenting all relevant information, the template enables patients to make informed choices based on their preferences, values, and concerns.
- Legal and ethical compliance: A standardized Informed Consent Form demonstrates compliance with legal and ethical requirements, including informed consent requirements.
- Protection for healthcare providers: The template provides a documented record of the informed consent process, protecting healthcare providers in case of any legal or ethical disputes.
- Enhanced communication and patient-provider relationship: Using a structured consent form promotes effective communication between healthcare practitioners and patients, encouraging open dialogue and fostering a trusting relationship between the patient and the provider.
- Standardization and efficiency: The template streamlines obtaining informed consent by providing a structured format. This saves time for healthcare providers and ensures consistent delivery of crucial information to patients.
- Documentation and patient safety: It serves as a reference for future care, ensuring that healthcare providers have a record of the patient’s consent, understanding, and any specific concerns or preferences expressed during the process.
Why use Carepatron as your Informed Consent Form app?
Carepatron is a cloud-based platform that allows users to create, store, and manage informed consent forms. It is a HIPAA-compliant platform that offers a variety of features that make it the best place to create and manage Informed Consent Forms.
One of the best features of Carepatron is its ability to create custom Informed Consent Forms. create forms that are tailored to Users can their specific needs. The platform offers a variety of consent templates that can be used as a starting point, and users can add or remove sections as needed. It is strongly advised that the informed consent document be reviewed by an attorney before implementation.
Another great feature of Carepatron is its ability to store Informed Consent Forms in the cloud. This makes it easy to access forms from anywhere and helps protect the privacy of the information contained in the forms.
Carepatron also offers a variety of features that make it easy to manage Informed Consent Forms. Users can track the status of forms, send reminders to patients, and collect signatures electronically.
Overall, Carepatron is a comprehensive platform offering various features Informed Consent Forms. It is a HIPAA-compliant platform that is easy to use and offers a variety of customization options.
Here are some of the specific reasons why Carepatron is the best place to do this type of work:
- HIPAA compliance: Carepatron is a HIPAA-compliant platform, which means it meets the strict security requirements set forth by the Health Insurance Portability and Accountability Act. This ensures that the information in your Informed Consent Forms is protected from unauthorized access.
- Ease of use: Carepatron is easy to use, even for those who need to be tech-savvy. The platform has a user-friendly interface makes it simple to create, store, and manage Informed Consent Forms.
- Customization options: Carepatron offers a variety of customization options that allow you to create Informed Consent Forms tailored to your specific needs.
- Affordability: Carepatron is an affordable option for creating and managing Informed Consent Forms. The platform offers a variety of pricing plans to fit your budget.
If you are looking for a HIPAA-compliant, easy-to-use, and affordable platform for creating and managing Informed Consent Forms, then Carepatron is the best option.
.png)
Commonly asked questions
Informed consent is typically obtained by healthcare providers, researchers, or authorized individuals who know and understand the treatment, procedure, or study. They are responsible for providing the necessary information and answering questions to ensure the individual's understanding.
An informed consent form should include details about the purpose of the treatment or study, potential risks and benefits, alternatives, confidentiality measures, financial considerations (if applicable), and the voluntary nature of consent. It should also allow the individual's signature to acknowledge their understanding and agreement.
The individual can withdraw informed consent anytime during the treatment, procedure, or study. Individuals can change their minds or discontinue participation without any negative consequences to their future care or treatment.
Research studies often have additional requirements for informed consent, particularly involving human subjects. These requirements may include providing information about the study's purpose, procedures, risks, benefits, confidentiality, voluntary participation, and the right to withdraw. Institutional Review Boards (IRBs) or ethics committees oversee and ensure compliance with these requirements.