The best thing that you can do is to better explain what the research is for and what’s in it for the patient. You can do this through the form itself, or by conversing with the patient. If they still refuse to sign, well, that’s that. They have the right to refuse to participate.

Informed Consent Form - Medical Research
Get access to a free informed Consent Form for medical research. Download the free PDF template and sample now.
Informed Consent Form - Medical Research Template
Commonly asked questions
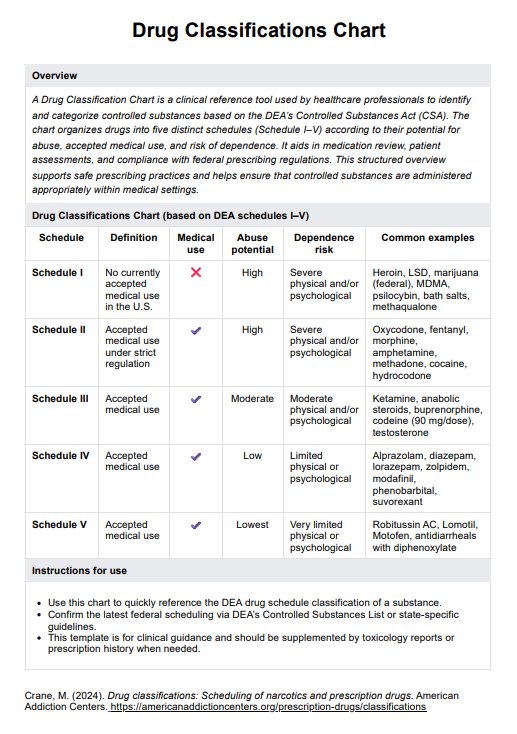
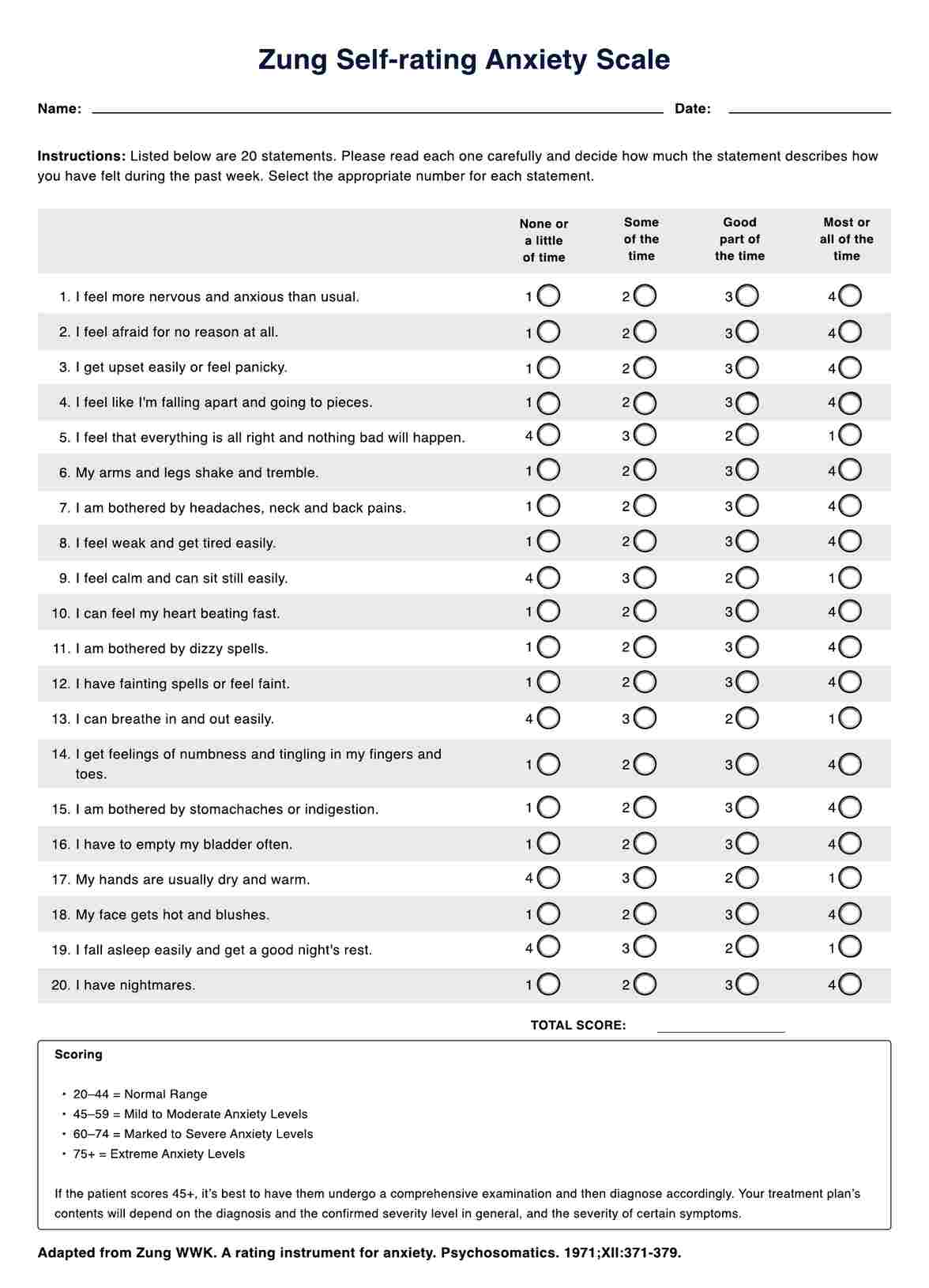
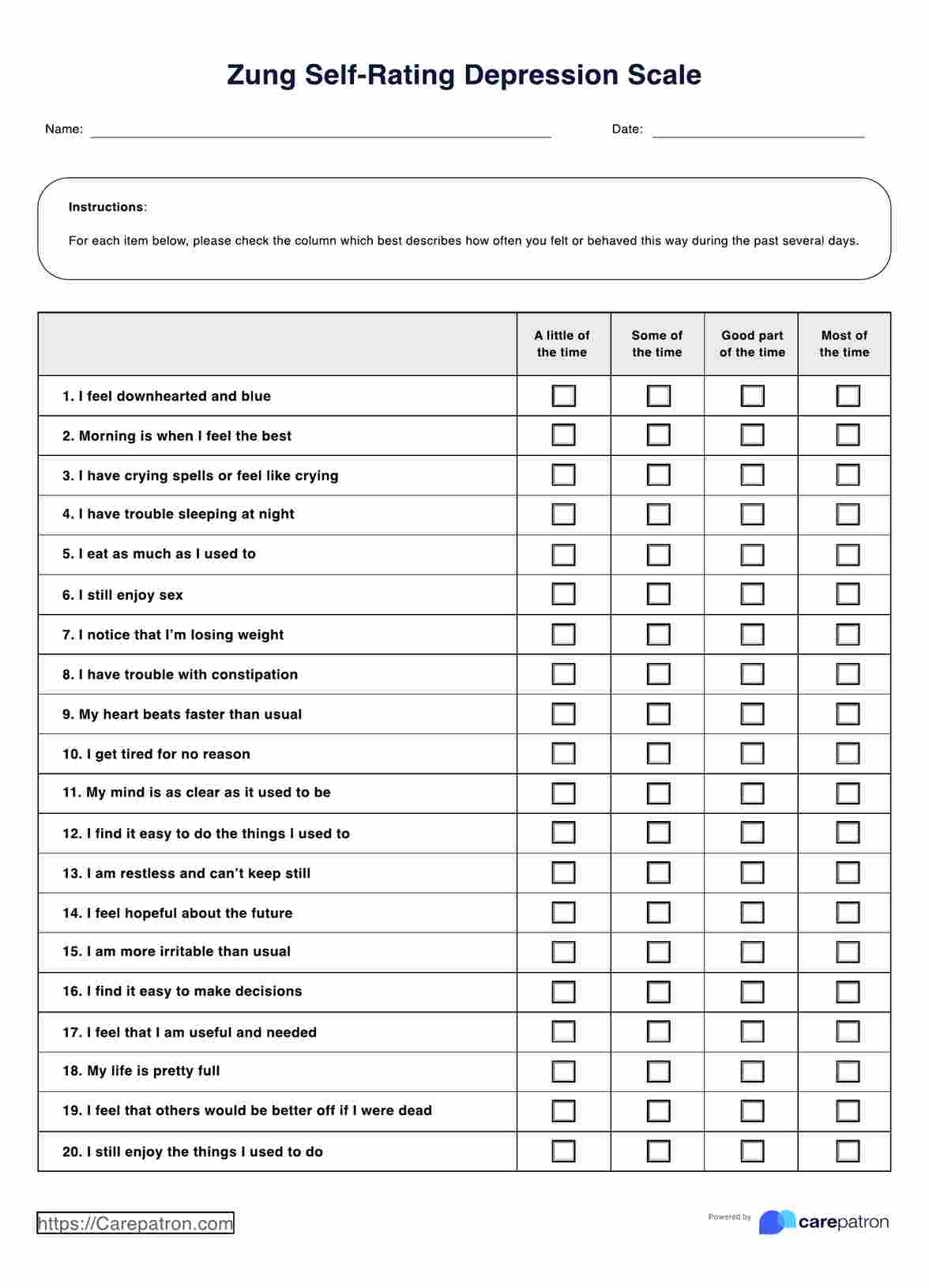
Informed Consent Forms in general are required, especially if it’s for medical procedures and treatment. All the more if these procedures and treatments carry the risk of death and other potential complications. The patient needs to know what they’re getting into.
Yes. Again, they have the absolute right to decide if they want to subject themselves to your research. Let’s say that you’re testing a new drug and they’ve consented to taking it, but they changed their mind because they suddently felt scared about the side effects. Well, you can’t really do anything about that. At the very least, you can still welcome them if they change their mind.
EHR and practice management software
Get started for free
*No credit card required
Free
$0/usd
Unlimited clients
Telehealth
1GB of storage
Client portal text
Automated billing and online payments