PDL1
The PDL1 Test is a crucial tool in cancer care. Explore its history, usage, and significance in personalized treatment.


What Is A PDL1 Test?
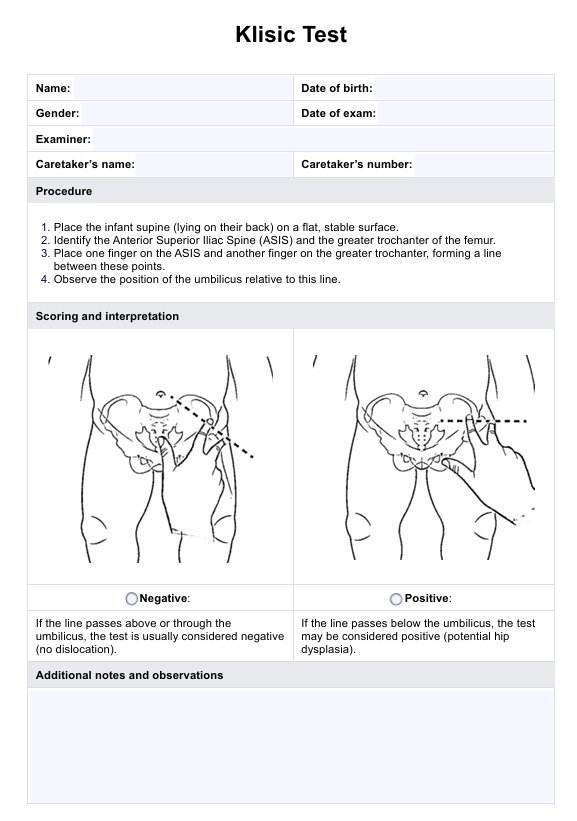
A PDL1 (Programmed Death-Ligand 1) test is a diagnostic procedure used to assess the presence and quantity of the PDL1 protein on cancer cells within a tumor tissue sample. This test plays a crucial role in determining whether a patient may benefit from a specific type of cancer treatment known as immunotherapy. PDL1 is a protein found in some healthy cells and cancer cells, serving as a "brake" that can inhibit the body's immune system response.
The test involves examining tumor tissue under a microscope using immunohistochemical staining to measure the percentage of tumor cells expressing PDL1. If high levels of PDL1 are detected, the tumor may be susceptible to immune checkpoint inhibitors, a class of immunotherapy drugs. These inhibitors block the PDL1 protein, allowing the immune system to target and fight cancer effectively.
PDL1 tests are critical for several cancer types, including lung, melanoma, and bladder. The results guide treatment decisions, with high PDL1 levels often leading to immunotherapy as the primary treatment option. However, it's essential to consult with a healthcare provider to determine the most appropriate treatment plan, as the role of PDL1 testing may vary depending on individual cases and evolving medical research.
PDL1 Template
PDL1 Example
How Does it Work?
Using the printable PDL1 Test form involves a straightforward process, ensuring that essential patient and medical information is accurately documented:
Step 1: Download the Form
Access the printable PDL1 Test form from a reputable medical source, clinic, or laboratory website. It is usually available as a downloadable PDF document.
Step 2: Patient Information
Begin by filling in the patient's full name, date of birth, gender, contact information (address, phone number, and email address), and emergency contact details.
Step 3: Medical History
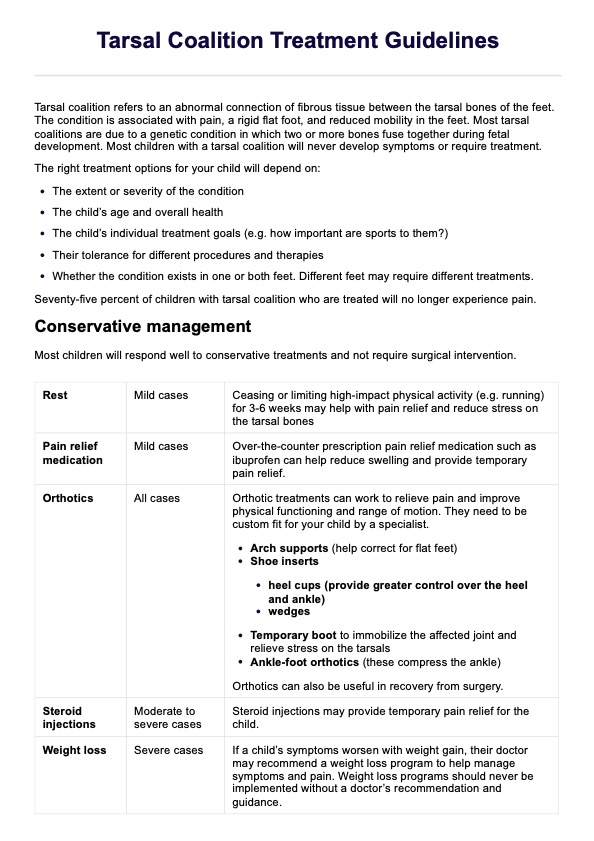
Document relevant medical history, including the diagnosis date, type of cancer, histology, stage at diagnosis, EGFR/ALK gene status, and other medical conditions. Be thorough and accurate.
Step 4: PD-L1 Testing
Indicate whether the patient has undergone PD-L1 testing before, provide the previous testing date (if applicable), and record the results if available. Specify if the doctor has recommended PD-L1 testing and if the patient knows PD-L1 levels.
Step 5: TMB Testing
Note whether Tumor Mutation Burden (TMB) testing has been suggested for the patient.
Step 6: Immunotherapy Considerations and Treatment Goals
Record whether the doctor has discussed immunotherapy as a treatment option, list specific immunotherapy drugs discussed, and outline the primary goals for cancer treatment.
When Would You Use This Test?
Oncologists and pathologists frequently employ this test when evaluating patients with cancer types, such as non-small cell lung cancer, melanoma, bladder cancer, and more. It is particularly useful when determining the potential eligibility of a patient for immunotherapy treatment. When considering an immunotherapeutic approach, the PDL1 test assists in identifying high levels of PD-L1 expression in tumor cells. This information is crucial, as patients with high PD-L1 levels may respond more favorably to immune checkpoint inhibitors, guiding practitioners to recommend immunotherapy as a primary treatment option.
In cases where the patient's medical history indicates the need for personalized cancer treatment plans, the PDL1 test is a valuable tool. It can be used to evaluate whether the patient's tumor exhibits sufficient PD-L1 expression to warrant immunotherapy. This is particularly relevant for patients who may not be eligible for other targeted therapies due to a lack of specific gene abnormalities. The test aids practitioners in making informed decisions regarding treatment strategies, enhancing the precision of cancer care.
The PDL1 test can also benefit patients undergoing routine check-ups or those with previous PD-L1 testing. Practitioners may recommend or conduct this test when considering changes in the patient's treatment plan, assessing the evolution of the disease, or exploring alternative therapeutic approaches. By ensuring that the test is used judiciously in appropriate clinical contexts, healthcare practitioners can offer patients the most effective and tailored treatment options for their cancer type.
What Do The Results Mean?
The results of a PDL1 test play a pivotal role in guiding cancer treatment decisions. When the test indicates high levels of PD-L1 expression in tumor cells (typically 50% or greater), it's considered a "positive" result. In this scenario, patients are prime candidates for immune checkpoint inhibitor immunotherapy, as the immune system is likely to respond effectively to treatment, increasing the chances of a successful outcome.
Conversely, if the PDL1 test shows low or negligible PD-L1 expression, it's a "negative" result. In such cases, the potential benefits of immunotherapy are limited, and alternative treatment options need to be explored.
Occasionally, the test may reveal intermediate PD-L1 levels, falling between high and low categories. These results require a more personalized approach, with healthcare providers carefully considering the patient's medical history and overall condition to determine the most appropriate treatment plan.
Understanding PDL1 test results is pivotal in tailoring cancer treatment to individual patients, ensuring the highest likelihood of success. Patients should have thorough discussions with their healthcare providers to interpret and act upon these results effectively, ensuring the best course of action for their unique cancer treatment journey.
Research & Evidence
The history of the PDL1 test is intricately linked to the evolution of cancer immunotherapy. The concept of programmed cell death (PD) pathways, including PD-L1, was initially explored in the late 1990s. Early research primarily focused on understanding how these pathways influenced immune responses and tolerance within the body. In 2002, the discovery of PD-1, a receptor molecule on immune cells, and its ligand PD-L1 marked a significant milestone.
Research intensified during the 2000s, unveiling the role of PD-L1 in tumor evasion from immune surveillance. Studies demonstrated that certain cancer cells exploit PD-L1 to deactivate immune responses, allowing them to thrive. This pivotal discovery prompted the development of immune checkpoint inhibitor drugs targeting the PD-1/PD-L1 pathway.
Clinical trials followed for these drugs, such as pembrolizumab (Keytruda) and nivolumab (Opdivo). The use of the PDL1 test as a companion diagnostic tool emerged, helping identify patients most likely to respond to these immunotherapies. Subsequent research has consistently shown that patients with high PD-L1 expression on their tumor cells are more responsive to PD-1/PD-L1 inhibitors.
The PDL1 test's historical journey from basic research to a critical clinical tool underscores its significance in modern oncology. Its role as an evidence-based predictor of immunotherapy success continues to evolve, shaping personalized cancer treatment strategies.
References
- Dong H., Strome S. E., Salomao D. R., et al. (2002). Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature Medicine, 8(8), 793-800.
- Hodi F. S., O'Day S. J., McDermott D. F., et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. The New England Journal of Medicine, 363(8), 711-723.
- Herbst R. S., Soria J. C., Kowanetz M., et al. (2014). Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature, 515(7528), 563-567.
Commonly asked questions
It is typically requested by oncologists and healthcare providers when evaluating cancer patients, especially those with lung cancer, melanoma, bladder cancer, and other malignancies where immunotherapy may be a treatment option.
These are used when healthcare providers need to assess a patient's eligibility for immunotherapy, helping determine if they are likely to respond positively to immune checkpoint inhibitors.
The tests involve analyzing a tumor tissue sample to measure the presence and quantity of the PD-L1 protein in cancer cells. This is typically done through immunohistochemical staining.