Therapeutic Drug Monitoring
Optimize patient care with Therapeutic Drug Monitoring. Ensure medication effectiveness and safety. Learn more.


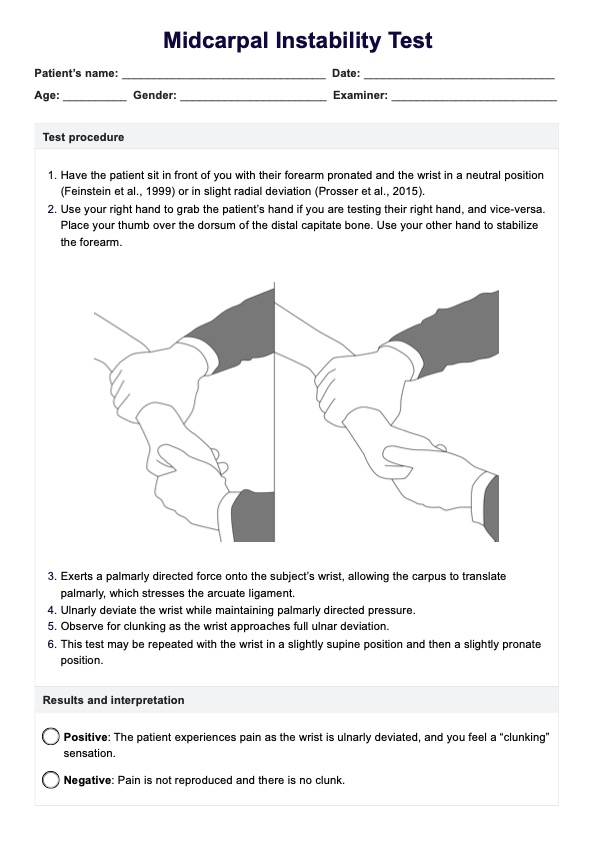
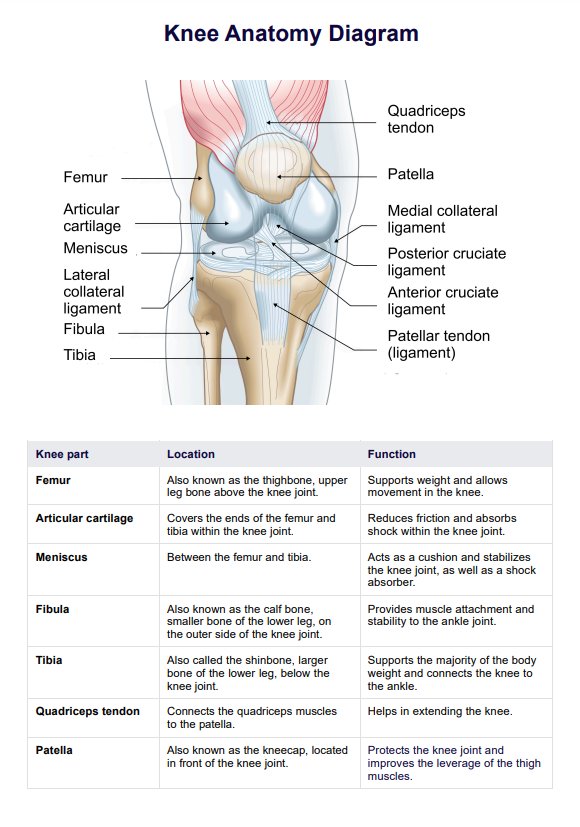
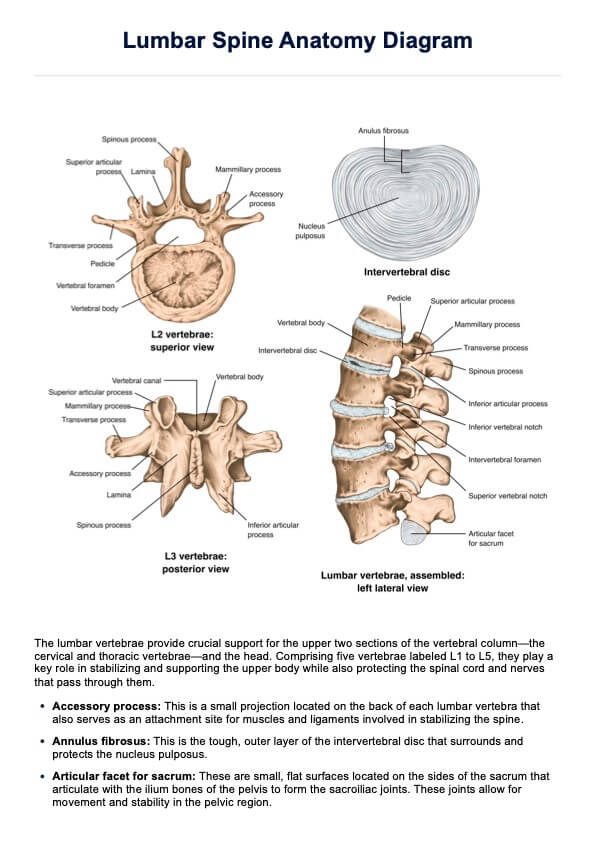
What Is Therapeutic Drug Monitoring?
Therapeutic Drug Monitoring (TDM) is an essential practice in modern healthcare that helps healthcare practitioners optimize patient care by ensuring that medications are administered at the correct dose and in a way that is safe and effective for each individual.
This process involves regularly measuring drug concentrations in a patient's blood, urine, or bodily fluids to assess their response to a specific medication. TDM serves as a crucial tool in tailoring treatment to meet the unique needs of each patient.
The primary objectives of Therapeutic Drug Monitoring are:
- Personalized Medicine: TDM allows healthcare professionals to customize medication regimens based on a patient's unique factors, such as age, weight, metabolism, and underlying medical conditions. This personalized approach maximizes the therapeutic benefits while minimizing potential side effects.
- Safety: By closely monitoring drug levels, TDM helps identify and prevent adverse drug reactions or toxicities when a medication is administered at doses that are too high or too low for an individual.
- Treatment Efficacy: TDM helps assess whether a medication works as intended. It is especially valuable when treating conditions with a narrow therapeutic window, where even slight fluctuations in drug levels can impact treatment outcomes.
- Drug Interactions: TDM can help healthcare practitioners manage complex medication regimens by ensuring that multiple drugs do not interact negatively. This is particularly important for patients taking multiple medications for various health conditions.
- Patient Compliance: It can also confirm that patients are taking their medications as prescribed.
Therapeutic Drug Monitoring Template
Therapeutic Drug Monitoring Example
How Does it Work?
TDM involves regular measurement of drug concentrations in a patient's bodily fluids, typically blood, to guide treatment. These are the key steps include analysis and monitoring of the drug levels:
Clinical Evaluation
The process begins with a thorough clinical assessment of the patient, considering factors such as age, weight, medical history, and underlying health conditions. This assessment helps determine the most suitable medication and dosage.
Choosing the Right Drug
Based on the patient's clinical profile, the healthcare practitioner selects the appropriate medication for the treated condition.
Prescription and Dosing
The healthcare practitioner prescribes the selected medication at a specific dose and frequency, often based on the patient's characteristics.
Collection of Bodily Fluids
Regularly scheduled samples of the patient's bodily fluids (most commonly blood) are collected at specified intervals to measure drug concentrations.
Sample Testing
The collected samples are sent to a clinical laboratory, where they undergo precise analysis to determine the drug's concentration in the patient's bloodstream.
Analysis Results
The laboratory results are interpreted in light of the patient's clinical status, the drug's therapeutic range, and any potential interactions with other medications.
Dosing Modifications
Based on the analysis, healthcare practitioners may adjust the medication dose to ensure drug concentrations are within the desired therapeutic range. This helps optimize the drug's efficacy and safety for the patient.
Regular Follow-up
TDM is not a one-time process. It requires ongoing monitoring, with additional samples collected and analyzed at scheduled intervals to assess the effectiveness of the adjusted medication regimen.
Communication
Patients are educated about the importance of TDM, the significance of compliance with medication instructions, and the need for regular follow-up appointments to ensure their safety and well-being.
Carepatron simplifies the TDM process by offering a printable form that documents patient information, medication, and lab results. This enhances collaboration among healthcare providers and promotes accessibility in modern healthcare practices.
When Would You Use This Test?
Therapeutic Drug Monitoring (TDM) is a valuable tool utilized by healthcare practitioners across various medical specialties to ensure the safe and effective administration of medications. TDM is particularly relevant in the following scenarios:
- Medications with a Narrow Therapeutic Range: TDM is crucial when managing drugs with a narrow therapeutic range, where minor deviations in drug levels can have a significant impact on treatment effectiveness and safety. Common examples include anticoagulants like warfarin and immunosuppressants used in organ transplant patients.
- Medication Dosage Individualization: TDM is employed when tailoring medication dosages to meet the unique needs of each patient. It is beneficial when patient characteristics, such as age, weight, genetics, or comorbid conditions, can affect drug metabolism and response.
- Complex Medication Regimens: In cases where patients are on multiple medications or have complex treatment regimens, TDM helps healthcare practitioners ensure that these drugs do not interact negatively. This is common in managing chronic diseases like HIV or in psychiatric care.
- Assessment of Therapeutic Response: TDM is used to assess how well a patient is responding to a medication. If a drug is not achieving the desired therapeutic effect, TDM can guide adjustments to the dosage or even a switch to an alternative medication.
- Prevention of Toxicity: TDM is essential to monitor drug levels and prevent potential toxicity, especially in cases where certain drugs have a narrow margin of safety, such as some antibiotics and antiepileptic medications.
- Compliance Monitoring: TDM is employed to verify patient compliance with medication regimens. Regular monitoring can reveal whether patients are taking their medications as prescribed.
- Changes in Clinical Status: When a patient's clinical status changes, such as during pregnancy or with the onset of a new medical condition, TDM may adjust medication dosages accordingly.
What do the Results Mean?
Interpreting TDM results is essential for healthcare practitioners to make informed decisions about a patient's medication regimen. Here are common TDM results and their significance:
- Low Drug Concentrations: Low drug levels in the blood may indicate the need for a higher dose to achieve the desired therapeutic range.
- Optimal Drug Concentrations: Therapeutic levels indicate that the drug concentration falls within the desired therapeutic range. This suggests that the medication is adequate and the patient will likely experience the desired therapeutic outcomes.
- High Drug Concentrations: Supratherapeutic levels signify the drug concentration is above the recommended therapeutic range. This can increase the risk of adverse effects or toxicity, and the healthcare practitioner may consider reducing the dose or discontinuing the medication.
- Lowest Concentration Before Next Dose: Trough levels are measured just before the next dose is administered. Low trough levels may indicate that the patient's drug levels drop too low between doses, potentially leading to reduced efficacy. Adjusting the dosing frequency or increasing the dose may be necessary.
- Highest Concentration After Dose: Peak levels are measured at the highest point after a dose. High peak levels may be indicative of a risk of toxicity. Dose adjustment or changes in the dosing interval may be necessary to maintain safe and effective treatment.
- Balanced Levels Over Time: Steady-state drug levels indicate that the drug concentration remains relatively constant over the dosing interval. This is often the goal for medications that require consistent therapeutic levels for optimal effect.
- No Detectable Drug: Non-detectable levels mean that the drug is not present in the patient's blood. This could be due to non-compliance or issues with drug absorption.
- Altered Levels Due to Interaction: TDM can reveal changes in drug levels caused by interactions with other medications. In such cases, adjustments to the medication regimen or choosing alternative drugs may be necessary.
Research & Evidence
Therapeutic Drug Monitoring (TDM) originated in the mid-20th century, as healthcare practitioners recognized the imperative need for a method to optimize medication dosages. It initially gained prominence in treating diseases such as tuberculosis, where monitoring blood levels of antituberculosis drugs played a pivotal role in ensuring therapeutic efficacy while averting toxicity.
The foundation of TDM is firmly rooted in pharmacokinetics, a subfield of pharmacology that investigates how drugs are absorbed, distributed, metabolized, and excreted within the body. Research in pharmacokinetics has significantly advanced the understanding of how drugs interact with the human body and has facilitated the development of TDM as a tool for individualized medicine.
Over the years, numerous clinical trials and observational studies have been conducted to establish the therapeutic ranges and optimal dosages for a wide range of medications (Ghiculesco, 2008). These studies have provided empirical evidence to guide healthcare practitioners in TDM.
Technological advancements have revolutionized the field of TDM, particularly through the development of high-performance liquid chromatography (HPLC), mass spectrometry, and immunoassays. These technologies have enabled precise measurement of drug concentrations in bodily fluids, greatly enhancing the accuracy and efficiency of TDM.
TDM has become a standard practice in various medical specialties, including infectious diseases, psychiatry, nephrology, and oncology. Improved patient outcomes evidence the utility of TDM, reduced adverse effects, and the ability to individualize treatment (Gis, 2020).
Professional medical organizations, such as the Clinical and Laboratory Standards Institute (CLSI) and the American Association for Clinical Chemistry (AACC), have developed guidelines and recommendations for TDM (Office of the Commissioner, 2021). These resources serve as a reference for healthcare practitioners and laboratories, ensuring the implementation of best practices in TDM.
References
Ghiculesco, R. (2008). Abnormal laboratory results: Therapeutic drug monitoring: which drugs, why, when, and how to do it. Australian Prescriber, 31(2), 42–44. https://doi.org/10.18773/austprescr.2008.025
Gis. (2020, August 10). Therapeutic Drug Monitoring - Gastrointestinal Society. Gastrointestinal Society. https://badgut.org/information-centre/a-z-digestive-topics/therapeutic-drug-monitoring/
Office of the Commissioner. (2021, August 12). Analysis of therapeutic drug monitoring in drug labels. FDA. https://www.fda.gov/science-research/fda-stem-outreach-education-and-engagement/analysis-therapeutic-drug-monitoring-drug-labels
Commonly asked questions
Healthcare practitioners, such as doctors and pharmacists, typically request Therapeutic Drug Monitoring (TDM) for patients who are on medications that require close monitoring, such as those with a narrow therapeutic range or complex dosing regimens.
TDM is used when optimizing medication dosages is essential. It's employed when managing medications with a narrow therapeutic range, ensuring patient safety, and personalizing treatment regimens to individual needs.
TDM involves regular measurements of drug concentrations in a patient's bodily fluids, typically blood. These measurements guide healthcare practitioners in adjusting medication dosages to maintain therapeutic levels, improve efficacy, and minimize potential side effects.