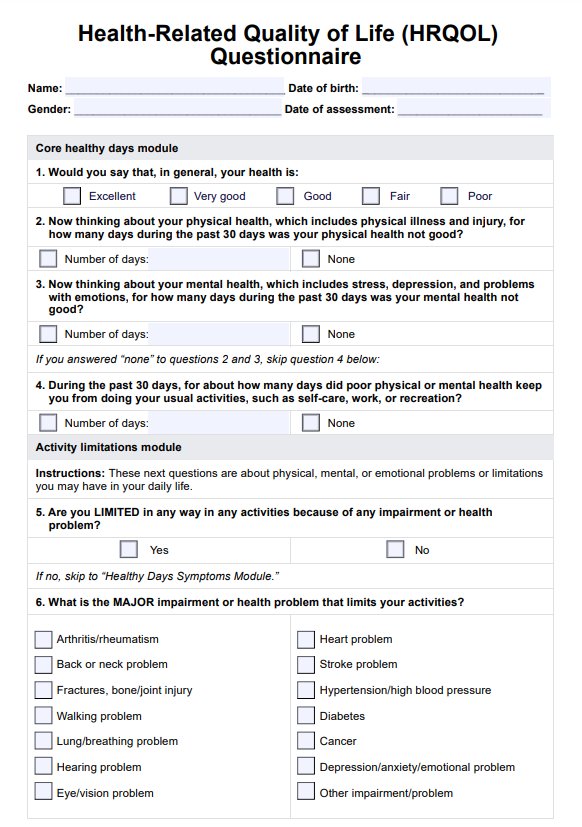
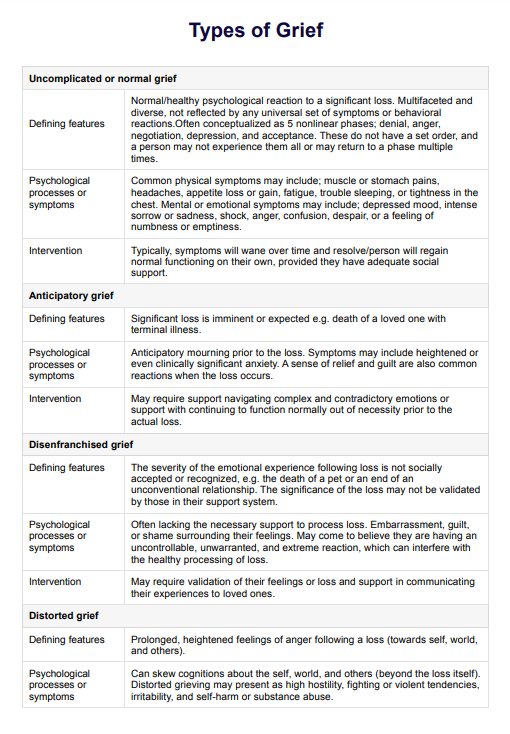
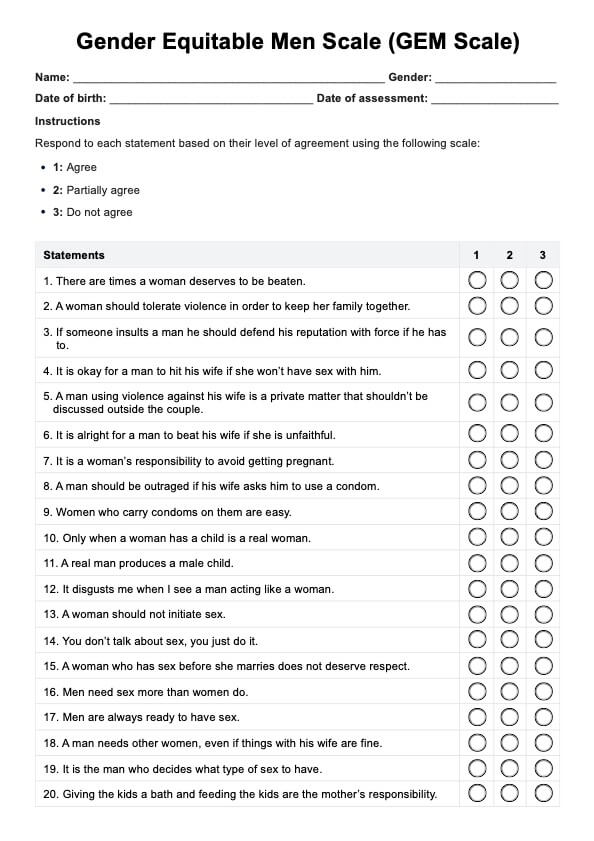
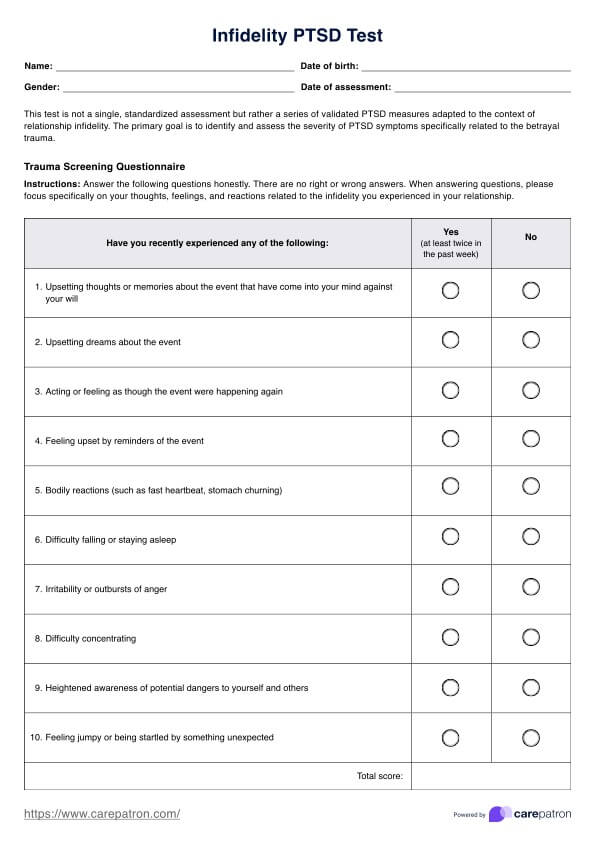
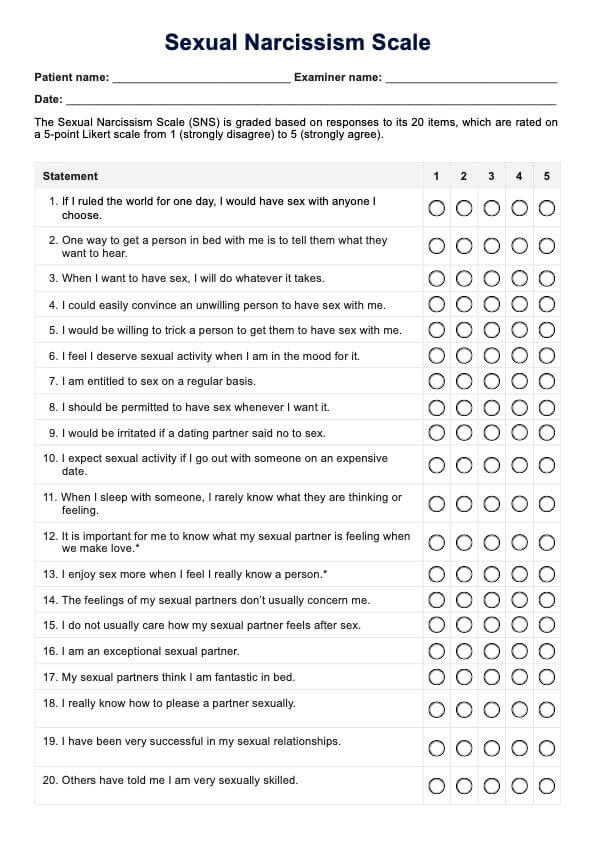
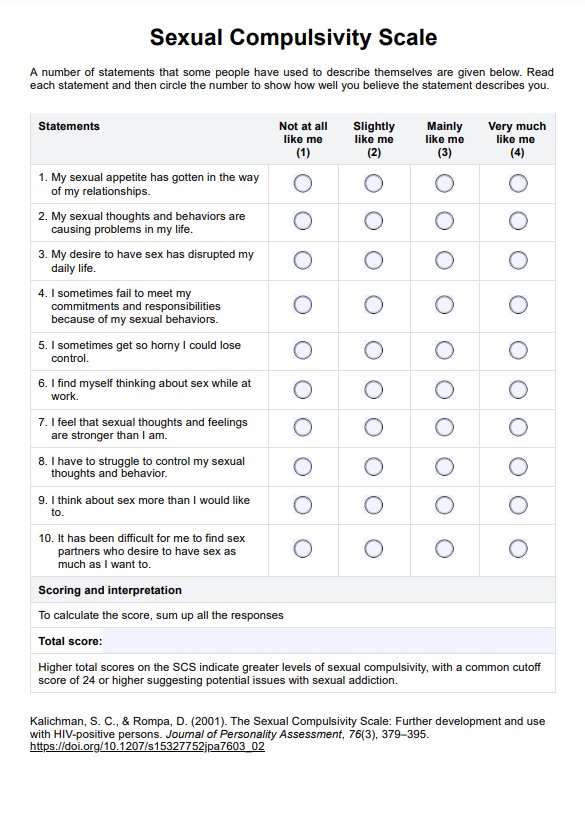
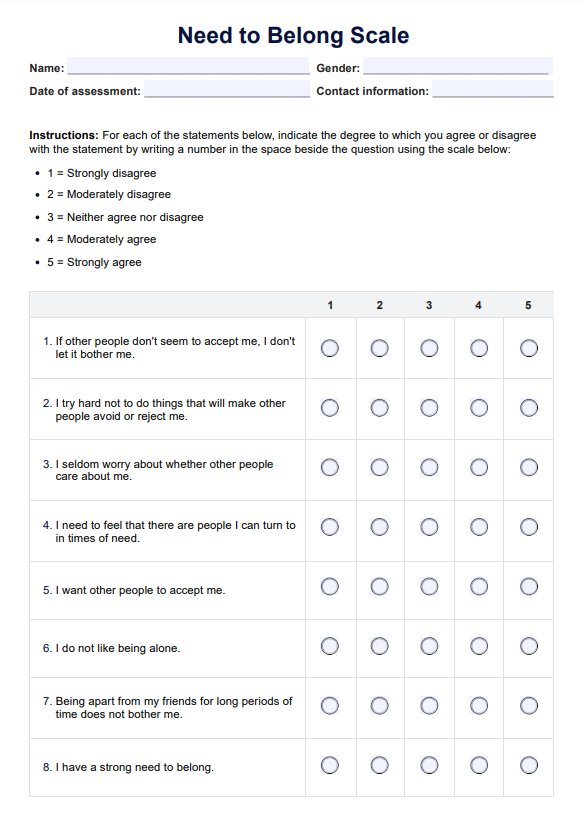
BCR-ABL1 Genetic Test
Discover the BCR-ABL1 genetic test for diagnosing and monitoring chronic myeloid leukemia and acute lymphoblastic leukemia. Learn more today.


What is a BCR-ABL1 Genetic Test?
The is a pivotal diagnostic instrument in the medical field, specifically in diagnosing and monitoring certain types of leukemia. This test is designed to identify the presence of the BCR-ABL1 fusion gene. This complex genetic marker is frequently associated with chronic myeloid leukemia (CML) and specific forms of acute lymphoblastic leukemia (ALL).
The ability to pinpoint this fusion gene plays an essential role in not only confirming a diagnosis of these diseases but also in determining the phase or stage of the disease in the patient's body. This information is crucial as it directly influences the treatment plan and can significantly affect the patient's prognosis.
Furthermore, the BCR-ABL1 genetic test is also employed as an ongoing monitoring tool during treatment. It helps medical professionals track the effectiveness of the treatment being administered and make necessary adjustments to ensure optimal patient outcomes.
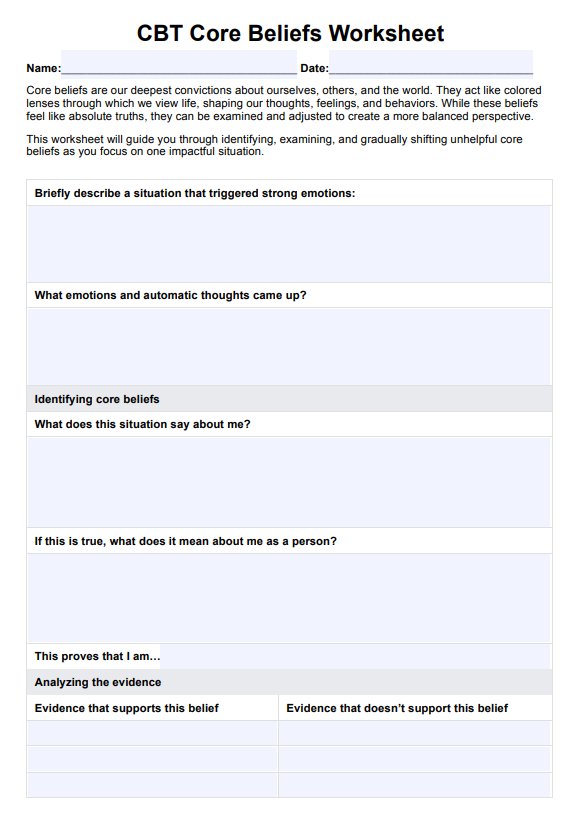
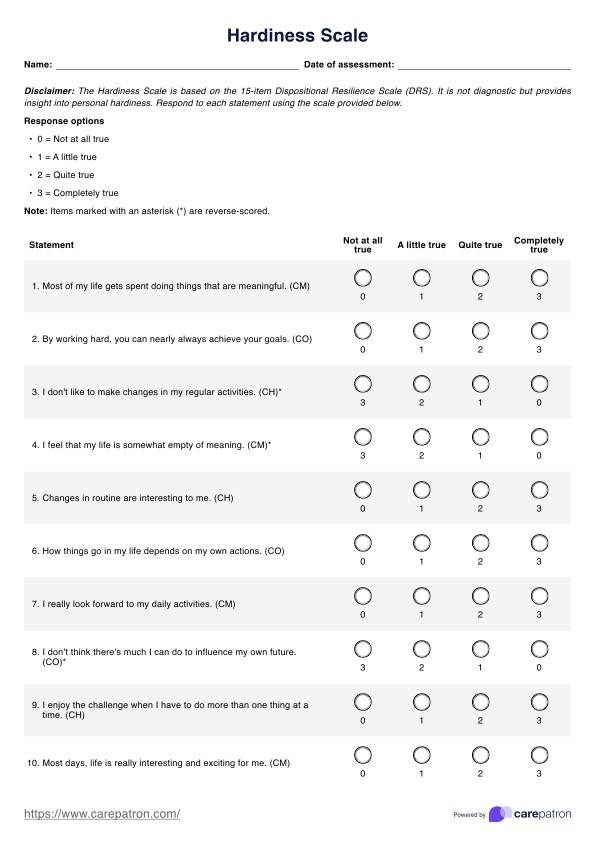
BCR-ABL1 Genetic Test Template
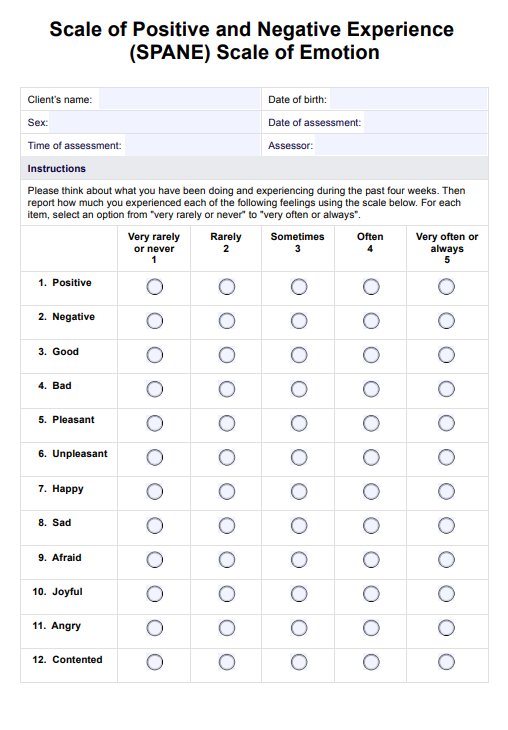
BCR-ABL1 Genetic Test Example
How does it work?
Understanding the step-by-step process of the BCR-ABL1 genetic test can provide valuable insight into its functionality and effectiveness. This test, designed to detect the BCR-ABL1 fusion gene, involves a detailed and meticulous procedure. The steps involved in this diagnostic process include:
Step 1: Sample Collection
The first step in the BCR-ABL1 genetic test is the collection of a sample from the patient. This typically involves drawing a blood sample or, in some cases, a bone marrow sample. This sample is then prepared for further testing. The collection process is usually quick and causes minimal discomfort to the patient.
Step 2: DNA Extraction
Once the sample has been collected, the next step is DNA extraction. In this phase, DNA is carefully extracted from the cells present in the collected sample. This isolated DNA will then serve as the basis for subsequent testing and analysis.
Step 3: PCR Amplification
Following DNA extraction, the target DNA sequence, specifically the BCR-ABL1 gene, is amplified using a technique known as Polymerase Chain Reaction (PCR). This process increases the quantity of the target DNA, making it easier to detect and analyze in the subsequent stages of the test.
Step 4: Detection
The final step in the BCR-ABL1 genetic test is detection. In this stage, the previously amplified DNA is detected and quantified. If present, this process identifies the BCR-ABL1 fusion gene and provides valuable information regarding the patient's condition.
A report detailing the findings is generated at the end of this process. We have a printable BCR-ABL1 genetic test for a practical understanding.
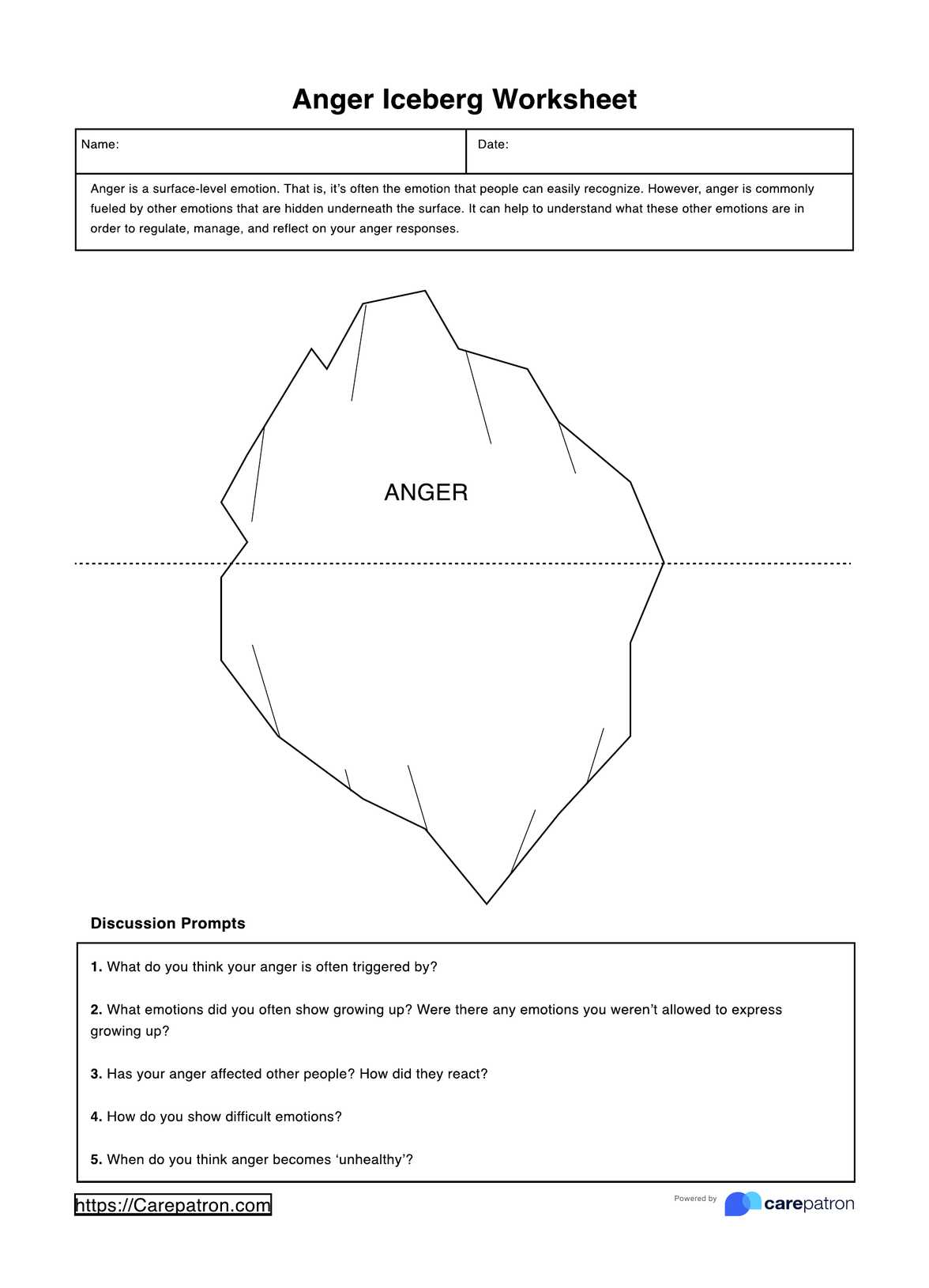
When would you use this test?
The BCR-ABL1 genetic test is a specialized diagnostic tool important in specific medical scenarios. It's essential to understand when it's appropriate to deploy this resource to ensure its effective use.
Primarily, a healthcare provider would order a BCR-ABL1 genetic test when there is a suspicion that a patient may have chronic myeloid leukemia (CML) or certain types of acute lymphoblastic leukemia (ALL). These suspicions could arise due to a combination of factors, such as the patient's symptoms, physical examination results, or findings from other laboratory tests. Symptoms leading to such suspicions could include fatigue, weight loss, night sweats, or an enlarged spleen.
The BCR-ABL1 genetic test does not just serve a diagnostic purpose; it's also a crucial tool for monitoring disease progression. By quantifying the amount of the BCR-ABL1 fusion gene, healthcare providers can determine the phase of the disease and track how it evolves. This information is invaluable in shaping the treatment plan and making necessary adjustments.
Additionally, the BCR-ABL1 genetic test is used to monitor the patient's response to treatment. By comparing the quantities of the BCR-ABL1 fusion gene before and after treatment, healthcare providers can gauge the effectiveness of the treatment strategy and make informed decisions about future course of action.
The BCR-ABL1 genetic test is a vital resource for healthcare practitioners dealing with CML or ALL. Its use is particularly appropriate when diagnosing these diseases, tracking their progression, and monitoring the response to treatment.
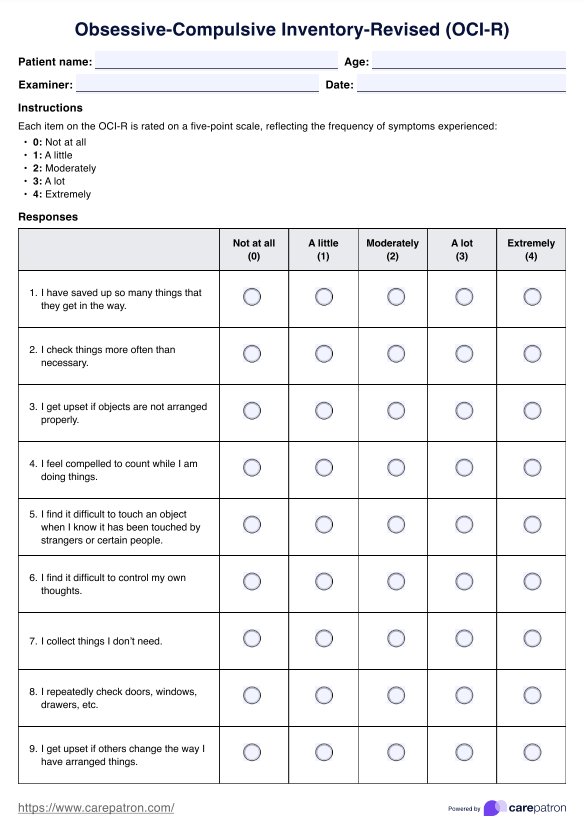
What do the results mean?
Understanding the implications of your BCR-ABL1 genetic test results is crucial in managing your health. Here's what those results generally signify.
A positive result on the BCR-ABL1 genetic test means that the BCR-ABL1 fusion gene was detected in the sample. This detection strongly indicates the presence of chronic myeloid leukemia (CML) or certain types of acute lymphoblastic leukemia (ALL). However, it's important to note that a positive result does not definitively diagnose these conditions; it simply raises the possibility of their presence, warranting further diagnostic testing and clinical evaluation.
The ratio of BCR-ABL1 to control genes, as provided in the test report, offers valuable insights into the disease's severity and progression. A higher ratio could suggest a more advanced stage of the disease or a less-than-optimal response to treatment. Conversely, decreasing this ratio over time often indicates a favorable response to therapy.
It's also worth noting that a negative result does not entirely rule out the presence of CML or ALL. It simply means the BCR-ABL1 fusion gene was undetected in the tested sample. Other tests may be necessary to confirm the diagnosis based on the patient's symptoms and clinical history.
Interpreting the BCR-ABL1 genetic test results should always be done with other diagnostic procedures and clinical findings. The results provide a piece of the puzzle but should be considered within the larger context of the patient's overall health status.
We have a Free BCR-ABL1 Genetic Test available for a comprehensive understanding.
Research & Evidence
The BCR-ABL1 genetic test has a solid foundation in medical research, with extensive studies corroborating its effectiveness in diagnosing and monitoring chronic myeloid leukemia (CML) and certain types of acute lymphoblastic leukemia (ALL).
The genesis of this test stems from the groundbreaking discovery of the Philadelphia chromosome in the late 1950s. This abnormal chromosome, resulting from a translocation between chromosomes 9 and 22, leads to the formation of the BCR-ABL1 fusion gene. This fusion gene is a characteristic feature of CML and some types of ALL, making it a crucial biomarker for these diseases.
Over the years, research has continually reinforced the value of the BCR-ABL1 genetic test. Numerous studies have highlighted its accuracy in detecting the presence of the BCR-ABL1 fusion gene. Moreover, research has demonstrated that quantifying this gene can provide important insights into disease severity and progression.
A landmark study underscored the significance of monitoring BCR-ABL1 levels in patients receiving tyrosine kinase inhibitor therapy for CML. The study found that tracking these levels could predict treatment response and survival outcomes.
Furthermore, guidelines from reputable health organizations like the National Comprehensive Cancer Network (NCCN) and the European LeukemiaNet (ELN) recommend the use of the BCR-ABL1 genetic test for diagnosing and monitoring CML and ALL. These guidelines lend substantial credibility to the test's utility in clinical practice.
The BCR-ABL1 genetic test stands on a robust platform of research and evidence. Its pivotal role in diagnosing and managing CML and certain ALL types is well-supported by many scientific studies and clinical guidelines.
References
- Nowell, P. C. (2007, August 1). Discovery of the Philadelphia chromosome: a personal perspective. Journal of Clinical Investigation; American Society for Clinical Investigation. https://doi.org/10.1172/jci31771
- BCRAB - Overview: BCR/ABL1, p210, mRNA Detection, Reverse Transcription-PCR (RT-PCR), Quantitative, Monitoring Chronic Myeloid Leukemia (CML), Varies. (n.d.). https://www.mayocliniclabs.com/test-catalog/Overview/89007
- BAKDM - Overview: BCR/ABL1, Tyrosine Kinase Inhibitor Resistance, Kinase Domain Mutation Screen, Sanger Sequencing, Varies. (n.d.). https://www.mayocliniclabs.com/test-catalog/Overview/89609
- Hochhaus, A., Baccarani, M., Silver, R. T., Schiffer, C. A., Apperley, J. F., Cervantes, F., Clark, R. E., CortǸs, J. E., Deininger, M. W., Guilhot, F., Hjorth?Hansen, H., Hughes, T. P., Janssen, J., Kantarjian, H. M., Kim, D. W., Larson, R. A., Lipton, J. H., Mahon, F., Mayer, J., . . . Hehlmann, R. (2020, March 3). European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia; Nature Portfolio. https://doi.org/10.1038/s41375-020-0776-2
Commonly asked questions
Healthcare providers, such as hematologists and oncologists, typically request this test when they suspect a patient has CML or ALL.
They're used for diagnosis, determining disease phase, and monitoring response to treatment in patients with suspected or confirmed CML or certain types of ALL.
The test is used to detect the presence of the BCR-ABL1 fusion gene in a blood or bone marrow sample.
The time it takes to get results can vary, but it's generally within a few days to a week.

.jpg)






-template.jpg)
















































-chart.jpg)