Informed consent is typically obtained by healthcare providers, researchers, or authorized individuals who know and understand the treatment, procedure, or study. They are responsible for providing the necessary information and answering questions to ensure the individual's understanding.

Informed Consent Form
Ensure clarity and transparency with our concise, informed consent form, empowering your clients to make informed decisions regarding their participation.
Informed Consent Form Template
Commonly asked questions
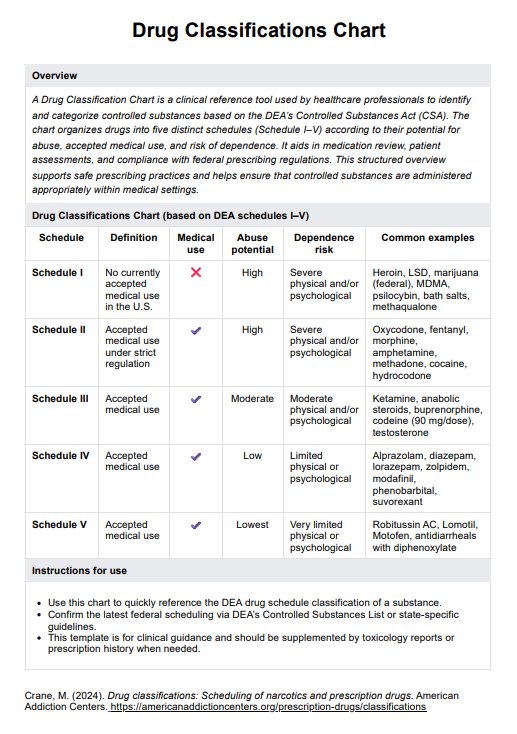
An informed consent form should include details about the purpose of the treatment or study, potential risks and benefits, alternatives, confidentiality measures, financial considerations (if applicable), and the voluntary nature of consent. It should also allow the individual's signature to acknowledge their understanding and agreement.
The individual can withdraw informed consent anytime during the treatment, procedure, or study. Individuals can change their minds or discontinue participation without any negative consequences to their future care or treatment.
EHR and practice management software
Get started for free
*No credit card required
Free
$0/usd
Unlimited clients
Telehealth
1GB of storage
Client portal text
Automated billing and online payments